Beer is the result of a multi-step brewing process, including a fermentation step, usually using one specific strain of yeast. Acidity is one of the flavors of beer, it measures how intense the taste of the beer is, and it can also give the beer a smooth, refreshing mouthfeel. At the same time, it is one of the conditions to ensure the smooth progress of brewhouse and fermentation.
Beer is an alcoholic beverage with a unique flavor made from wort fermented by brewer’s yeast. The unique flavor of beer is produced and determined by the metabolites of yeast in the wort.
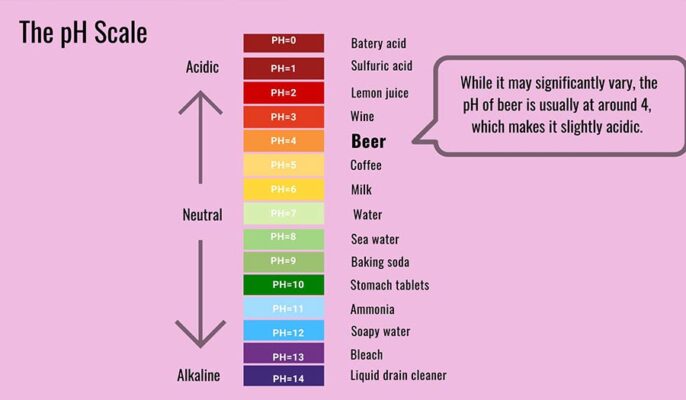
Is beer acidic or alkaline?
Beer is acidic. Whether you drink beer for its color, smell, bitterness, texture or taste. Fermentation converts the sugar into alcohol and carbon dioxide, making the beer more acidic.
Most popular beers produced in the United States have a pH between 4.0 and 5.0, which is in the middle of the acidity range. As you might expect, sour beers are a bit more acidic.
Sometimes the color of a beer indicates how acidic it is. For example, darker roasted malt produces more acid, which raises the pH of the beer.
Certain types of bacteria or yeast used in beer, such as lactic acid bacteria, can also make beer more sour.
The pH of beer also affects the following factors:
- Appearance of beer
- Taste (hop bitterness)
- Beer foam stability
- Extraction Potential
- Hot break formation
- Hops Oil Extraction
Acid in beer
It is generally believed that beer has an average pH of 4, which makes it acidic. Most of the acids in beer are organic acids, the most common being acetic acid (vinegar flavor), pyruvic acid, lactic acid (yogurt flavor), malic acid and citric acid (strong sour flavor). They originate from raw materials, wort boiling and yeast metabolism. Lactic and acetic acids may also arise from unwanted or uncontrolled microbial contamination.
Source of sour in beer
Malt
Acids in malt can be divided into acid reactions produced by biochemical reactions acid and glycation. The acid in malt is phosphate, followed by methampiosic acid, acetic acid, propionic acid, citric acid, senior fatty acid and various amino acids. At the beginning of the glycation, the organic phosphate contained in malt is hydrolyzed by malt phospreen, forming a buffer composed of phosphate and acid phosphate.
Water
Calcium and magnesium ions in the water can convert alkaline K2HPO4 in the malleon juice into acidic KH2PO4, which increases the acidity of the malt juice and reduces the pH value.
Add acid
During the preparation of the malt, the acidity of the water may be added due to the lack of acid in the malt or the alkali of the brewing water is too high. This is to regulate the pH of the ocean juice and condense enzymes and proteins in malt. Adding more acids can also increase the activity of enzymes to the greatest extent, reduce the color of malt and beer, and increase the immersion rate of malt.
Acids of fermentation
During the fermentation process, yeast uses fermented sugar for metabolism. The metabolism of yeast not only produces ethanol, water, and CO2, but also volatile acids such as acetic acid, butyl acid, and non -volatile acids such as lactic acid, amber acid. The main volatile acid is acetic acid, which is the most volatile acid that has the greatest impact on beer acid. Amber acid is the least volatile acid produced during beer fermentation and has a great impact on beer acid. Some carbon dioxide produced during the fermentation process will dissolve in beer and combine with water to form weak carbonate.

Ways to Control Acid in Beer
Control raw and auxiliary materials
The raw material should be high-quality, mildew-free barley, and the germination temperature of the barley should be controlled. Since malt will carry various bacteria to varying degrees during transportation and storage, it must be sterilized during storage.
Control of the brewhouse process
During the brewhouse process, priority is given to adjusting the water quality of the brewing water to cut the amount of acid added. If the pH of the brewing water is high, the pH can be adjusted by adding lactic acid malt or using biological acidification technology. Also, the saccharification time should be shortened as much as possible, and the filtered wort should be clear and transparent.
Selection of yeast strains
Less acid-producing yeasts are used in the fermentation process. Strengthen the management of yeast in the production process, and avoid the use of yeast that contaminates wild yeast and miscellaneous bacteria. Also, it is also necessary to avoid excessive temperature fluctuations in the fermenter, discharge the yeast in time, and prevent the yeast from autolyzing.
Controlling the fermentation process
Control the fermentation process (fermentation temperature, yeast addition, aeration, etc.) to cut acid rise.
Hygienic Control and Management of Brewing Equipment
- Determine the microbiological testing points, and take regular samples for inspection.
- All pipelines and storage tanks are cleaned according to regulations. After each CIP cleaning, the laboratory must be notified to take a sample and test it. It can be used only after passing the test.
- Remove the filter and clean it once a week; every time the fermenter is filled, the cold wort pipes and plate heat exchanger need to be cleaned with a hot caustic cycle.
- Replace the filter with cardboard and perform CIP cleaning.
- Each nozzle should be disinfected.